(p. A1) BOSTON — Like thousands of children in the U.S., Maggie Leaver has short bowel syndrome. These children can’t absorb enough nutrients from food, and some need intravenous feedings to survive.
A baby’s digestive system can adapt over time, but that may take months or years. Many of these babies can’t wait. For reasons not fully understood, children put on intravenous nutrition may suffer liver damage. Some require liver and small bowel transplants, risky procedures that don’t always work. Others die waiting for a transplant.
In July, in a paper in the scientific journal Pediatrics, researchers at Children’s Hospital Boston reported on a small study that suggested a promising treatment. They found that by switching from the standard intravenous formula to a different kind — called Omegaven — babies weren’t progressing to liver failure. Omegaven, used in Europe for adults, isn’t approved in the U.S. and is considered experimental treatment.
"The kids aren’t dying anymore," says Mark Puder, a pediatric surgeon who was lead investigator on the study. "We think we have a good treatment."
But Dr. Puder’s effort to get Omegaven widely used in babies has put him in an unusual conflict with the German company that developed the drug. Fresenius Kabi AG, which makes Omegaven, says it isn’t interested in bringing the drug to the U.S. market. The company says it doesn’t agree that Omegaven is the best drug for these babies and has a new product that it believes is better.
In 28 of 29 babies treated with Omegaven so far at Children’s Hospital, Dr. Puder says they were able to stop further liver damage — and damage that children already incurred seemed to improve. Some babies who were switched to Omegaven rebounded enough that they were taken off the waiting list for an organ transplant. At one point, Maggie Leaver’s condition deteriorated so much that her surgeon thought she was going to die. Now the 18-month-old is thriving at home in Hingham, Mass.
. . .
Mr. Ducker says the company’s new product, called SMOFlipid, "presents a better option for pediatric feeding." The company believes the new product does contain all the essential fatty acids babies need and can be used on its own.
Fresenius Kabi says it doesn’t want to invest the resources required to test both products for approval by the U.S. Food and Drug Administration. It hopes to eventually sell the new product in the U.S., Mr. Ducker says, although no timetable has been set and no trials are under way.
. . .
Because Omegaven is considered experimental in the U.S., if hospitals want to try it, they have to ask permission from the FDA for each individual patient. The FDA has regulations that enable doctors to use experimental drugs in certain (p. A15) emergency situations.
If hospitals obtain the required permissions, they must then find a way to buy the drug on their own, since insurers typically won’t cover Omegaven because it’s experimental. The cost can run from $50 to $100 a day per patient. At Children’s Hospital Boston, the surgical department has already spent close to $100,000 to buy Omegaven for babies.
. . .
Dr. Mooney says he wrestled almost from the beginning about whether to put Maggie on Omegaven. He knew about Dr. Puder’s results, which he calls "amazingly great," but the number of children treated was still small. He worried about adverse effects. "It is so easy to get caught in the hype of new things," Dr. Mooney says. Maggie was already fragile. What if he put her on Omegaven, he says, "and there was a horrible side effect that could tip her over the edge?"
But when standard therapies failed, he felt "there was nothing else to do." Given that the treatment is experimental, Dr. Mooney says he believes it was right to wait. But he also feels Omegaven has made a difference. "Five years ago, every single one of the kids taking Omegaven would be dead by now, Maggie included," he says.

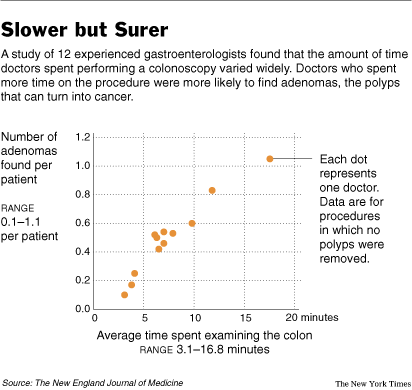 Source of graph: online version of the NYT article cited below.
Source of graph: online version of the NYT article cited below.
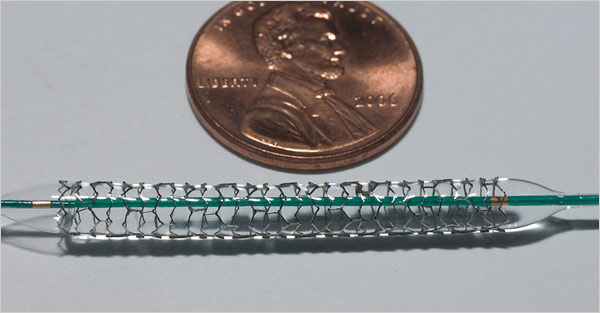 A stent. Source of photo: online version of the 3/27/07 NYT article cited below.
A stent. Source of photo: online version of the 3/27/07 NYT article cited below. A dental grill, one form of the hip-hop jewelry sometimes called "bling-bling." Source of image:
A dental grill, one form of the hip-hop jewelry sometimes called "bling-bling." Source of image: 
 Harvard Medical School antiaging researcher Dr. David Sinclair. Source of image:
Harvard Medical School antiaging researcher Dr. David Sinclair. Source of image: