 A CVS pharmacy MinuteClinic. Source of photo: online version of the WSJ article cited below.
A CVS pharmacy MinuteClinic. Source of photo: online version of the WSJ article cited below.
It’s Friday evening and you suspect that your child might have strep throat or a worsening ear infection. Do you bundle him up and wait half the night in an emergency room? Or do you suffer through the weekend and hope that you can get an appointment with your pediatrician on Monday — taking time off your job to drive across town for another wait in the doctor’s office?
Every parent has faced this dilemma. But now there are new options, courtesy of the competitive marketplace. You might instead be able to take a quick trip on Friday night to a RediClinic in the nearby Wal-Mart or a MinuteClinic at CVS, where you will be seen by a nurse practitioner within 15 minutes, most likely getting a prescription that you can have filled right there. Cost of the visit? Generally between $40 and $60.
These new retail health clinics are opening in big box stores and local pharmacies around the country to treat common maladies at prices lower than a typical doctor’s visit and much lower than the emergency room. No appointment necessary. Open daytime, evenings and weekends. Most take insurance.
Much like the response to Hurricane Katrina, private companies are far ahead of the government in answering Americans’ needs, this time for more accessible and more affordable health care. Political leaders across the country seeking to expand government’s role in health care should take note.
For the full commentary, see:
GRACE-MARIE TURNER. "Customer Health Care." The Wall Street Journal (Mon., May 14, 2007): A17.

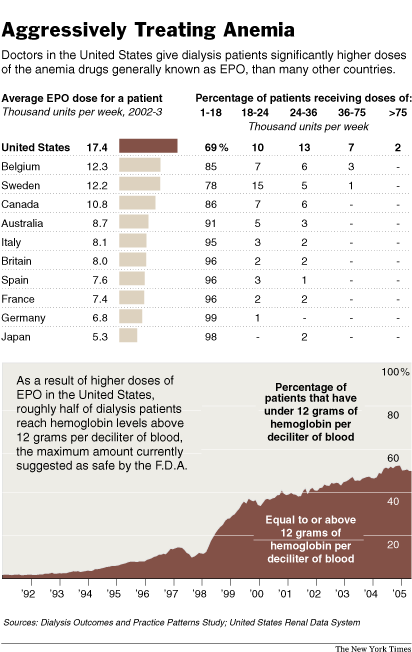 Source of graphic: online version of the NYT article quoted and cited below.
Source of graphic: online version of the NYT article quoted and cited below. 

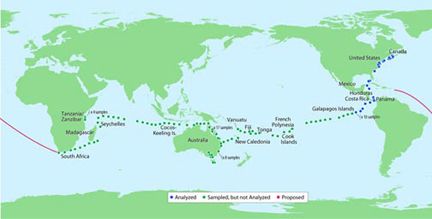 The projected path of Venter’s Sorcerer II ship in collecting sea organisms. Source of map:
The projected path of Venter’s Sorcerer II ship in collecting sea organisms. Source of map: 


